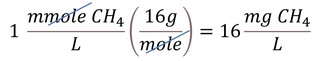The concentration of a chemical in a water body is often expressed in mass per unit volume, e.g., 1 milligram of nitrate (NO3-) per liter of water, or 1 mg/L. Since 1 L of fresh water has a mass of 1 kg, a concentration of 1 mg/L is equivalent to 1 mg/kg, so it is also referred to as 1 part-per-million or ppm (since there are a million milligrams in a kilogram). Likewise, 1 ug/L (one microgram per liter) is equivalent to 1 ppb (part-per-billion).
For molecules such as NO3-, concentrations can either include the mass of the entire compound (e.g., 1 mg NO3-/L) or just the mass of the relevant atom (e.g., 1 mg NO3--N/L, which should be understood as 1 mg of N in the form of NO3- per liter of water). Concentrations can also be expressed in molar units, which can be converted to mass-based concentrations using the molecular weight. For example, methane (CH4) has a molecular weight of 16 g/mole, so 1 mmole/L CH4 is equivalent to 16 mg/L, as shown in the equation below.